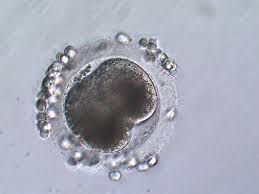
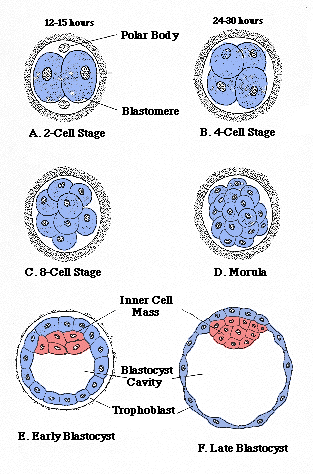
In the Intracytoplasmic Sperm Injection procedure, an oocyte, or egg, is collected from a donor mare’s follicle prior to ovulation and cultured until maturity. Once mature, the oocyte is fertilized in a laboratory via micro-injection of a single sperm cell. The fertilized oocyte should start to divide to become an embryo with the first cell division occurring 24-30 hours after sperm injection. The blastocyst stage is reached after seven to 10 days of culture in an incubator. (see last image below for stages of fertilization) The embryo can then be transferred to the uterus of a recipient mare, shipped to another facility for transfer or frozen for future use.
Common utilizations of ICSI occur in mares with persistent fertility problems preventing embryo production and in stallions with limited sperm availability. Since only a single sperm cell is injected, ICSI can utilize very small quantities of frozen semen.
WHAT TO EXPECT FROM THE ICSI PROCESS
Recovery of an oocyte from a dominant follicle can be expected on approximately 75% of attempts. Immature oocytes can often be collected from smaller follicles at the same time. Oocytes fertilized by Intracytoplasmic Sperm Injection typically divide and grow on 70-80% of attempts, and of those, 20-30% will grow in culture to the blastocyst stage for non-surgical transfer.
Oocyte quality and vigor tend to decline with the donor mare’s age, so older donors typically have lower success rates than younger mares. Oocyte numbers can be a limiting factor as well―some mares have many small follicles each cycle which facilitate the recovery of multiple oocytes, while others have only one or two follicles present in each cycle.
On average, a donor mare will require three cycles to establish a pregnancy. This can vary as some mares establish multiple pregnancies in their first cycle, while others with decreased oocyte viability may prove to be more challenging.
HOW ICSI WORKS
OOCYTE COLLECTION
Oocytes can either be recovered just prior to ovulation from the large dominant follicle each cycle or from the smaller subordinate follicles at any time. Most commonly we collect from both the dominant and subordinate follicles at the same time each cycle. A typical mare will have one dominant and 7-8 subordinate follicles each cycle. The average oocyte collection rate is about 75% per follicle for the dominants and 60-70% for the subordinates giving an average yield of .7 oocytes from the dominant follicle and 5 from the subordinates per cycle. This is highly variable from mare to mare, as some mares have many subordinate follicles and some quite few; older mares tend to have somewhat fewer subordinate follicles. The oocyte from the dominant follicle is usually of higher quality but since the oocytes from the subordinate follicles are generally more numerous approximately 2/3 of the pregnancies produced come from these oocytes from the small follicles.
Oocytes are generally recovered as a cumulus-oocyte complex, an oocyte surrounded by the cumulus cells that nourish it, dislodged from the follicle wall. Initially as a follicle grows, the cumulus cells are tightly adhered to the follicle wall and the mass of cells itself is very dense; the cells are very close together. As the follicle grows and becomes a dominant follicle the cumulus mass starts to expand as the cells secrete an extracellular matrix that spreads them apart. Nearing ovulation this expansion makes the attachment to the follicle wall weaker and weaker so that during ovulation the oocyte can be swept from the follicle into the oviduct. The loosening of the attachment makes the oocyte somewhat easier to recover nearing ovulation, although as the follicle enlarges it is more difficult to create the turbulence necessary to dislodge the oocyte. For these reasons, aspiration of the immature follicles with their firmly attached cumulus-oocyte complex, tend to have the highest recovery rate when they are between 10 and 20 mm in size, while the dominant follicles are best aspirated less than 14 or 15 hours before an expected ovulation.
Semen used for ICSI can be fresh, fresh-cooled, or frozen, but frozen is most commonly used. Since only a few cells are needed, generally just the tip of a frozen semen straw (about 1/10th of a straw) is cut off to thaw. This leaves the other 90% frozen to use in the future. The cells are processed in several different ways, but nearly always use a “swim-up” to help select motile cells. This is performed by placing the sperm sample at the bottom of a tube of culture medium, allowing time for the spermatozoa to swim, and the sample is taken farther up the tube.
Individual sperma are selected under the microscope and immobilized by crushing the tail with the injection pipette. Once in the oocyte, this damage to the cell membrane covering the tail will facilitate the passage of “sperm factor” into the oocyte. Sperm factor is a chemical that is critical in activating the oocyte to start the process of cell division.
For the ICSI procedure itself suction on the holding pipette is used to position and stabilize the oocyte. The oocyte is positioned so that the injection will take place away from the polar body (extruded excess genetic material but near the genetic material still in the cell) and into an area of good visualization if possible.
Once the pipette enters the ooplasm (cytoplasm of the oocyte) some ooplasm is aspirated into the pipette to assure that the oolemma (oocyte cell membrane) has been completely penetrated and to help with oocyte activation. The oolemma is very elastic and can be difficult to puncture, even with the pipette inserted well into the oocyte. In this video there are two oocytes injected. In the first, the oolemma was pierced by the pipette itself and can be seen sliding back into its original position before the sperm was injected. The oolemma did not rupture spontaneously in the second oocyte so aspiration was used to pierce it. The oolemma can be seen being aspirated into the pipette before it ruptures and allows aspiration of ooplasm and the ejection of the sperm.
Once fertilized, oocytes are placed in culture in micro-droplets under a layer of mineral oil. They are maintained in an incubator with increased carbon dioxide and decreased oxygen concentrations to regulate proper temperature, pH, and to reduce oxidative damage. The mineral oil overlay prevents evaporation of the culture medium and helps decrease the chance of contamination.
When successfully fertilized and activated, an oocyte quickly starts making rapid and dramatic changes. One of the first events that can be visualized is extrusion of excess genetic material in the second polar body, often within 3-4 hours following fertilization.
Good quality ICSI produced early blastocsyts should have an approximately 80% pregnancy rate when transferred non-surgically to a recipient mare. The early embryonic loss of pregnancy is slightly higher in ICSI produced embryos than in naturally occurring pregnancies.